**Exploring Pfizer’s Paxlovid for COVID-19 Treatment**
**Introduction:**
Pfizer’s Paxlovid, a combination of two antiviral drugs, is a significant development in combating the COVID-19 virus. The medication has shown promise in keeping individuals with mild to moderate symptoms out of the hospital.
**Evolution of COVID-19 Treatments:**
Over the past four years, the landscape of COVID-19 treatments has transformed. This evolution has been driven by the diligent efforts of scientists and doctors who meticulously analyzed data to establish evidence-based protocols for patient care. Notably, the National Institutes of Health (NIH) COVID-19 Treatment Guidelines have played a pivotal role, garnering over 50 million views and serving as a global reference for healthcare professionals.
**Impact of Evidence-Based Guidelines:**
Dr. Rajesh Gandhi, an infectious diseases specialist at Massachusetts General Hospital and a member of the NIH’s COVID-19 Treatment Guidelines Panel, reflects on the uncertainties of treating COVID-19 in the early stages of the pandemic. During this period, unproven remedies like hydroxychloroquine and ivermectin were widely experimented with, despite later studies proving their ineffectiveness against the coronavirus.
**Collaborative Efforts in Guideline Development:**
The NIH assembled a panel of over 40 experts at the onset of the pandemic to formulate comprehensive treatment guidelines. Dr. Cliff Lane, director of the clinical research division at the National Institute of Allergy and Infectious Diseases (NIAID) and a panel co-chair, describes the continuous updates to the guidelines based on rigorous reviews of emerging scientific literature and data discussions.
By adhering to the English language keyphrase “COVID treatment guidelines, NIH,” healthcare providers can access invaluable resources for evidence-based care protocols.
The Era Comes to a Close
Reevaluating COVID Treatment Guidelines by NIH
Recently, the pace of introducing new COVID-19 treatments has significantly slowed down, leading the guideline committee to reassess their strategies. Lane mentioned, “Determining the ideal timing to conclude the guidelines was challenging. However, as the frequency of necessary meetings decreased, and we even had to cancel some of our regular calls, about six months ago, we initiated discussions on how to conclude the guidelines thoughtfully without leaving a gap.”
The most recent update of the NIH’s COVID-19 Treatment Guidelines dates back to February. The records of the COVID treatment guidelines, accessible online until August, illustrate the progression of scientific knowledge and technological advancements throughout the pandemic.
Lane emphasizes that moving forward, specialty doctors groups like the American College of Physicians and the Infectious Diseases Society of America will take charge of COVID-19 treatment guidelines. According to him, they typically oversee best-practice guidelines, making them the designated custodians of COVID treatment guidelines.
Panel members emphasize that the development of COVID-19 treatment guidelines provides valuable insights for managing future emerging infectious diseases, as highlighted by the NIH.
Evolution of COVID Treatment Guidelines
In the spring of 2020, as the initial wave of COVID-19 patients inundated hospitals across the U.S., medical professionals faced a steep learning curve regarding the progression of the disease. Dr. Gandhi from Massachusetts General Hospital recalls the uncertainty during that time, stating that the guidelines issued in April reflected the lack of clarity on effective treatments. However, through swift observations, particularly among hospitalized patients, effective treatment strategies began to emerge.
By June 2020, data analysis supported a treatment protocol for severely ill patients. This approach involved the use of steroids such as dexamethasone to mitigate the body’s immune response and the combination with antiviral medications to hinder viral replication.
About a year into the pandemic, a significant breakthrough occurred with compelling evidence indicating that early administration of lab-made antibodies could prevent COVID-19 patients from requiring hospitalization. Dr. Lane highlights the unexpected and impactful nature of this discovery, especially considering past unsuccessful attempts to develop antibody therapies for influenza.
Monoclonal antibodies, the class of drugs utilized, provided valuable insights into the virus itself, according to Dr. Phyllis Tien from the University of California, San Francisco, who is part of the COVID-19 treatment panel. Although initially effective, these antibodies targeted the rapidly mutating spike protein of the coronavirus. Consequently, new strains of the virus rendered each antibody version ineffective within approximately a year.
This dynamic approach of developing antibodies to combat the virus’s mutations proved to be unsustainable in the long run.

COVID Treatment Guidelines by NIH
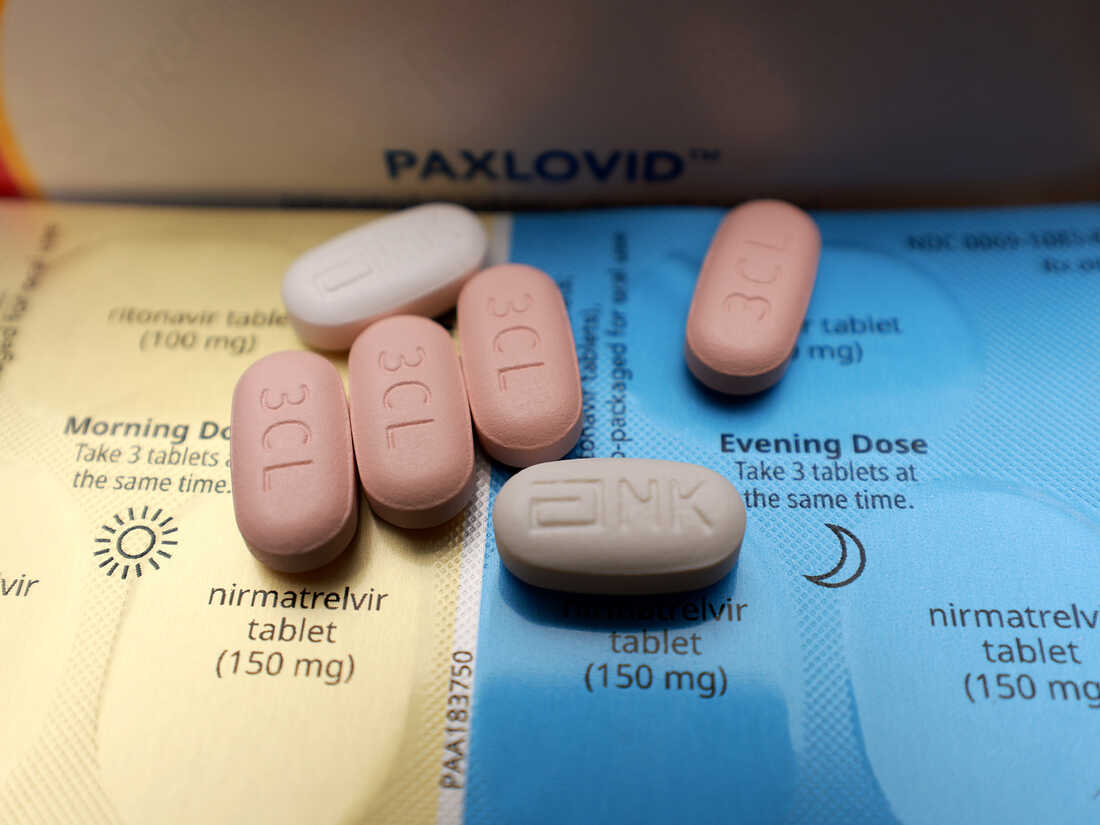
At the close of 2021, two courses of pills were greenlit by the Food and Drug Administration for COVID-19 patients to self-administer at home in hopes of recovery: Merck’s molnupiravir and Pfizer’s Paxlovid, a blend of two antiviral medications – ritonavir and nirmatrelvir.
Carl Dieffenbach, who heads the AIDS division at NIAID and contributes to the agency’s antiviral development initiative for pandemics, notes that both treatments have their drawbacks. He mentions that Molnupiravir’s efficacy is limited, while Paxlovid, although effective, poses challenges when combined with many common medications. Dieffenbach highlights that numerous healthcare providers are hesitant to oversee patients who require Paxlovid but are also on other medications like statins.
Remdesivir, another antiviral medication, is deemed quite effective in managing mild to moderate COVID-19 cases, albeit its intravenous administration makes it less accessible to patients. Gilead, the pharmaceutical company behind Remdesivir, attempted to convert it into a pill form, but unfortunately, the endeavor was unsuccessful.
Challenges in Implementing Effective COVID Treatment
According to Jenny Shen, a research scientist at the CUNY Institute for Implementation Science in Population Health, the obstacles associated with outpatient COVID treatment options have led to low utilization rates among eligible patients. Shen’s study revealed that during the peak of the pandemic, only 2% of individuals diagnosed with COVID-19 received molnupiravir, and 15% received Paxlovid, despite being suitable candidates for these medications.
Utilizing data from 2021-2022, which coincided with the period when the federal government procured these drugs from manufacturers and distributed them at no cost to various healthcare facilities, Shen highlights a decline in treatment uptake post late 2023. This decrease is attributed to the transition of these medications to the commercial market, resulting in reduced accessibility due to the elimination of free distribution and the introduction of copayments.
These findings shed light on the underutilization of recommended COVID treatment guidelines, as outlined by the National Institutes of Health (NIH).

Challenges in Implementing COVID Treatment Guidelines by NIH
Doctors may hesitate to prescribe outpatient treatments outlined in the COVID treatment guidelines by NIH due to their complexity, especially when patients have other health issues, notes Shen. Additionally, a significant obstacle lies in convincing patients with risk factors that they are susceptible to severe illness. Shen observes that patients often delay seeking treatment until their condition worsens, rendering the treatment less effective.
Despite the increasing hospitalization rates of around 13,000 individuals weekly due to COVID-19, enhancing patient education on the efficacy and timing of these drugs could empower sick individuals to make more informed choices, suggests Shen. Furthermore, Dieffenbach mentions a promising COVID-19 drug in advanced clinical trials developed by the Japanese company Shionogi. This pill course is being evaluated for its effectiveness against both acute and long-term COVID. Dieffenbach expresses anticipation for the outcomes of these trials, highlighting this as a significant development on the horizon.
For more information on COVID treatment guidelines, please visit our site 60time.com. Additionally, please don’t forget to follow us on social media at Facebook for more updates.